Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kitamura, Akira
JAEA-Data/Code 2020-020, 164 Pages, 2021/03
Part of JAEA's Thermodynamic Database (JAEA-TDB) for solubility and speciation of radionuclides (JAEA-TDB-RN) for performance assessment of geological disposal of high-level radioactive and TRU wastes has been updated with subsuming the database for geochemical calculations (JAEA-TDB-GC). This report has focused to update JAEA-TDB-RN after selecting change in standard Gibbs free energy of formation (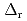
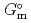 ), change in standard enthalpy change of formation (
), change in standard enthalpy change of formation (
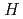

 ), standard molar entropy (
), standard molar entropy (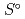
 ) and, heat capacity (
) and, heat capacity (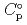 ), change in standard Gibbs free energy of reaction (
), change in standard Gibbs free energy of reaction (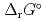
 ), change in standard enthalpy change of reaction (
), change in standard enthalpy change of reaction (
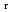
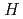

 ) and standard entropy change of reaction (
) and standard entropy change of reaction ( ) as well as logarithm of equilibrium constant (log
) as well as logarithm of equilibrium constant (log
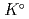 ) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
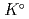 have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Kitamura, Akira; Yoshida, Yasushi*
Journal of Radioanalytical and Nuclear Chemistry, 327(2), p.839 - 845, 2021/02
Times Cited Count:3 Percentile:44.61(Chemistry, Analytical)Thermodynamic data for radium for radioactive waste management have been predicted using an electrostatic model and correlation with the ionic radii of the alkaline earth metals. Estimation of the standard Gibbs free energy of formation and standard molar entropy of aqueous radium species and compounds has been based on such approaches as extrapolation of the thermodynamic properties of strontium and barium, and use of a model of ion pair formation. The predicted thermodynamic data for radium have been compared with previously reported values.
 -UO
-UO
 -CO
-CO
 system and integration with JAEA's thermodynamic database for geochemical calculations
system and integration with JAEA's thermodynamic database for geochemical calculationsKitamura, Akira
JAEA-Data/Code 2018-018, 103 Pages, 2019/03
The latest available thermodynamic data were critically reviewed and the selected values were included into the JAEA-TDB for performance assessment of geological disposal of high-level radioactive and TRU wastes. This critical review specifically addressed thermodynamic data for (1) a zirconium-hydroxide system through comparison of thermodynamic data selected by the Nuclear Energy Agency within the Organisation for Economic Co-operation and Development (OECD/NEA), (2) complexation of metal ions with isosaccharinic acid based on the latest review papers. Furthermore, the author performed (3) tentative selection of thermodynamic data on ternary complexes among alkaline-earth metal, uranyl and carbonate ions, and (4) integration with the latest version of JAEA's thermodynamic database for geochemical calculations. The internal consistency of the selected data was checked by the author. Text files of the updated and integrated thermodynamic database have been prepared for geochemical calculation programs of PHREEQC and Geochemist's Workbench.